Sinopharm Vaccine Wirksamkeit / Fast Eine Million Chinesen Haben Coronaimpfung Erhalten
The inactivated virus approach has been used for decades against many different diseases so it is very well proven in the real world but it maybe slightly weaker than the newer approaches. SinoPharms inactivated coronavirus candidate.
Cnbg Vaccine Efficacy Rate Boosts Confidence Global Times
This trial is being conducted by Sinopharms China National Biotec Group CNBG in alliance with the.

Sinopharm vaccine wirksamkeit. China-based pharmaceutical company Sinopharm has initiated a Phase III clinical trial to assess its Covid-19 vaccine candidate in Abu Dhabi UAE. The interim recommendations and the background. Listing a study does not mean it has been.
Tuesday Chinas state-run Sinopharm said Chinese an inactivated COVID-19 vaccine developed by its subsidiary China National Biotec Groups. The Chinese vaccine is an inactivated virus basically a killed COVID virus while the Pfizer vaccine uses mRNA and the OxfordAstraZeneca uses a viral vector. Are safe are effective and.
This article provides a summary of the interim recommendations. It completed Phase III trials in Argentina Bahrain. It completed Phase III trials in Argentina Bahrain Egypt Morocco Pakistan Peru and the United Arab Emirates UAE with over 60000 participants.
The Sinopharm vaccine contains SARS-CoV-2 that has undergone treatment with a chemical called beta-propiolactone. A two-dose COVID-19 vaccine from Chinas Sinopharm was 504 effective in preventing infections in health workers in Peru when it was seeing a surge in cases fuelled by virus variants and booster. When the tests are completed the vaccine is.
Sinopharm is testing the potential vaccine in the United Arab Emirates in a Phase 3 trial expected to recruit 15000 people as China has too few new cases to be a useful trial site reuters. The Sinopharm vaccine has a self-reported efficacy rate of 79 percent which global health experts say is more than adequate but its data has not been publicly released. The vaccine made by Chinas Sinopharm was less effective than others at preventing infection hospitalization and death especially among people.
In SinoPharms case the company inactivated the coronavirus with beta. Sinopharm a unit of China National Pharmaceutical Group said Phase 3 trials for the vaccine have started at the international level. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine BIBP developed by SinopharmChina National Pharmaceutical Group.
BBIBP-CorV also known as the Sinopharm COVID-19 vaccine or BIBP vaccine is one of two inactivated virus COVID-19 vaccines developed by Sinopharms Beijing Institute of Biological Products sometimes written as Beijing Bio-Institute of Biological Products resulting in the two different acronyms BBIBP and BIBP for the same vaccine. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. People with moderately to severely compromised immune systems should receive an additional dose of mRNA COVID-19 vaccine after the initial 2 doses.
CDC does not recommend one vaccine over another. The candidate is said to be the worlds first inactivated vaccine to enter a Phase III trial. Reduce your risk of severe illness.
This chemical binds to the viruss genetic material and stops it from. Chinas state-owned Sinopharm said an inactivated COVID-19 vaccine developed by its subsidiary China National Biotec Groups Wuhan Institute. Sinopharm BBIBP-CorV also known as the Sinopharm COVID-19 vaccine is one of two inactivated virus COVID-19 vaccines developed by Sinopharm.
Now we have some clinical data on yet another category of vaccine. All currently authorized and recommended COVID-19 vaccines. Here is what you need to know.

Coronaimpfstoff Sinopharm Gibt Wirksamkeit Mit 79 Prozent An

Chinese Covid 19 Vaccine Far Less Effective Than Initially Touted In Brazil Wsj

Safety And Efficacy Of An Rad26 And Rad5 Vector Based Heterologous Prime Boost Covid 19 Vaccine An Interim Analysis Of A Randomised Controlled Phase 3 Trial In Russia The Lancet
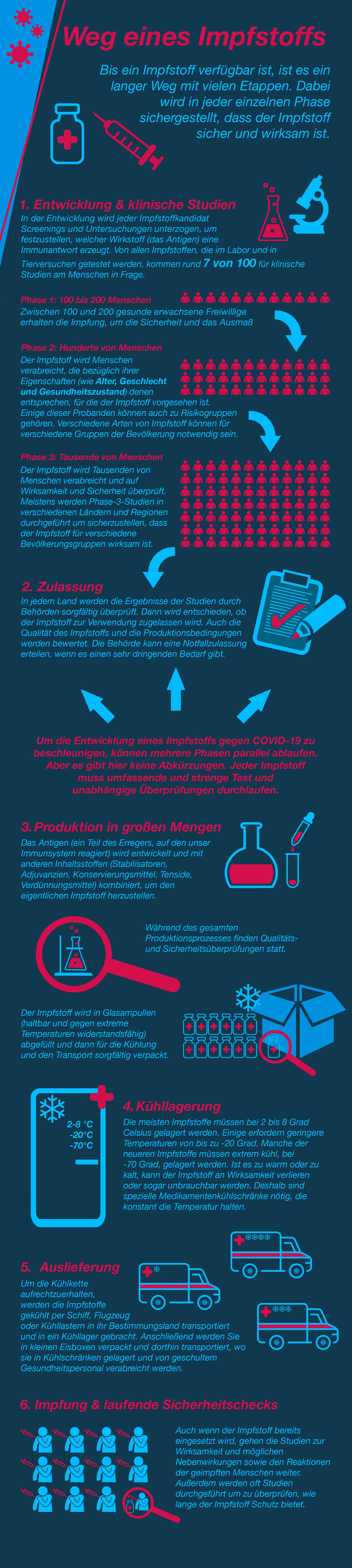
Covid 19 Impfungen Die Verschiedenen Impfstoffarten Erklart Global

New Covid Strain Sinopharm Vaccine Effective Against South African Variant News Khaleej Times

Safety And Immunogenicity Of Chadox1 Ncov 19 Vaccine Administered In A Prime Boost Regimen In Young And Old Adults Cov002 A Single Blind Randomised Controlled Phase 2 3 Trial The Lancet

Sinopharm Who Erteilt Erstem Chinesischen Corona Impfstoff Notfallzulassu Pz Pharmazeutische Zeitung

Coronavirus Wie Wirksam Sind Die Impfstoffe Aus China Wissen Umwelt Dw 01 02 2021

Coronavirus Wie Wirksam Sind Die Impfstoffe Aus China Wissen Umwelt Dw 01 02 2021

Verwirrung Um Wirksamkeit Chinesischer Impfstoffe Wissen Umwelt Dw 12 04 2021

Covid Vaccine From China S Sinopharm Is 86 Effective Says Uae Financial Times

Efficacy And Safety Of An Inactivated Whole Virion Sars Cov 2 Vaccine Coronavac Interim Results Of A Double Blind Randomised Placebo Controlled Phase 3 Trial In Turkey The Lancet

Dandago Uiras Nkoshi On Twitter Take Note The Following Vaccination Sites Are Open Today In Windhoek Only The Sinopharm Covid 19 Vaccine Is Available For Now Get Vaccinated And Save Lives Mhssnamibia Https T Co 9b413odfdd

Sinopharm Beim Corona Impfstoff Hinkt China Dem Westen Hinterher

Sinovac Brazil Results Show Chinese Vaccine 50 4 Effective Bbc News

Fast Eine Million Chinesen Haben Coronaimpfung Erhalten

Cnbg Vaccine Efficacy Rate Boosts Confidence Global Times

China Liefert Impfstoffe An Covax Impfallianz Aktuell Asien Dw 12 07 2021